Abstract
Introduction
Secondary acute myeloid leukemia (s-AML) includes AML with myelodysplasia-related changes (AML-MRC) transformed from an antecedent diagnosis of myelodysplastic syndrome (MDS) or from myeloproliferative and myelodysplastic overlap syndrome (MPN/MDS), as well as therapy-related AML (t-AML) arising in patients with prior exposure to leukemogenic therapies. S-AML has a dismal prognosis. Although the genomic profiling of AML has been well studied, few studies have focused on the molecular aberrations of s-AML. A recent study has demonstrated shared genomic aberrations by AML-MRC and t-AML including mutations in TP53, SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2. Another recent study has shown that AML patients with mutations in TP53 and chromatin-splicesosome confer poor prognosis and likely represent s-AML. As such, further characterization of the genomic landscape of s-AML is needed. Recurrent somatic PHF6 mutations are rare in de novo AML (~3%), but the frequency and characteristics of PHF6 mutated s-AML are largely unknown.
Patients and Methods
AML-MRC and t-AML patients were searched from the patient database at the MSKCC between 1/2014 and 12/2016. All the patients were reviewed and the diagnosis was confirmed. Only patients with genomic sequencing studies (a targeted NGS panel comprising 30 genes with mutations enriched in AML) performed at diagnosis were included.
Results
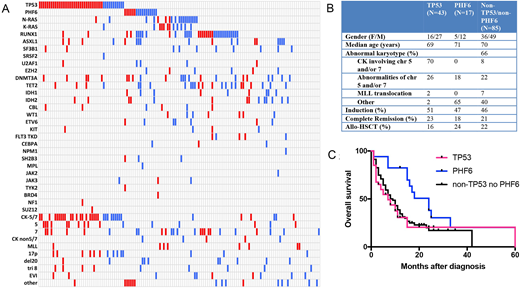
152 patients were identified. There were 7 t-AML patients with favorable cytogenetic abnormalities that likely represent a distinct biological subgroup; therefore these cases were excluded from further analysis. AML-MRC (N=75) and t-AML (N=70) patients had similar clinical characteristics including ages, gender distribution, cytogenetic risk, treatment and survival (median survival: 8 months in t-AML and 10 months in AML-MRC, p=0.4); The mutational profiles of AML-MRC and t-AML cohorts were also comparable with recurrent TP53, PHF6, RAS, and RUNX1 mutations, albeit with different frequencies (Figure 1; Red represents t-AML and blue AML-MRC). Therefore we combined AML-MRC and t-AML cohorts for further analysis.
43 (29.7%) patients had TP53 mutations, 17 (11.7%) patients had PHF6 mutations, and 85 (58.6%) patients had neither mutation (non-TP53/non-PHF6). TP53 and PHF6 mutations were mutually exclusive. TP53 mutations were nearly exclusively associated with adverse cytogenetic abnormalities including alterations involving chromosomes (chr) 5p and/or 7q (41/43, 95.3%) with the majority being complex karyotype (30/43, 70%). By contrast, only 18% PHF6 mutated s-AML patients had chr 5p/7q abnormalities and none of them had complex karyotype. Among the patients with non-TP53/non-PHF6 mutations, 30% had chr 5p/7q abnormalities and 6% had complex karyotype. Although secondary AML has very poor prognosis, PHF6 mutated patients had better overall survival than the other two groups (Log rank test, p=0.0095; median survival: PHF6 vs TP53 vs. non-TP53/non-PHF6: 24 vs. 7 vs. 9 months). Immunophenotypically, the blasts from PHF6 mutated patients showed a high frequency of loss of CD38 (44% vs 17%, p=0.04) and CD33 expression (69% vs 28%, p=0.004) compared to PHF6 unmutated cases, indicative of a less mature, stem-cell derived phenotype
Conclusion
PHF6 mutations define a unique subset of s-AML that is associated with a more primitive stem/progenitor immunophenotype, absent complex karyotype and relatively better outcomes. PHF6 mutations are mutually exclusive to TP53 mutations. Studies are needed to further define the genomic classification of s-AML patients in an unbiased way and to elucidate the role of PHF6 mutations in s-AML.
Goldberg:AROG: Research Funding; Abbvie: Research Funding; Pfizer: Research Funding; Celgene: Research Funding. Tallman:Orsenix: Other: Advisory board; AbbVie: Research Funding; Cellerant: Research Funding; BioSight: Other: Advisory board; AROG: Research Funding; Daiichi-Sankyo: Other: Advisory board; ADC Therapeutics: Research Funding. Arcila:Invivoscribe, Inc.: Consultancy, Honoraria. Levine:Loxo: Consultancy, Equity Ownership; Gilead: Honoraria; Roche: Consultancy, Research Funding; Epizyme: Patents & Royalties; Imago: Equity Ownership; Qiagen: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Research Funding; C4 Therapeutics: Equity Ownership; Novartis: Consultancy; Janssen: Consultancy, Honoraria; Isoplexis: Equity Ownership; Prelude: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal